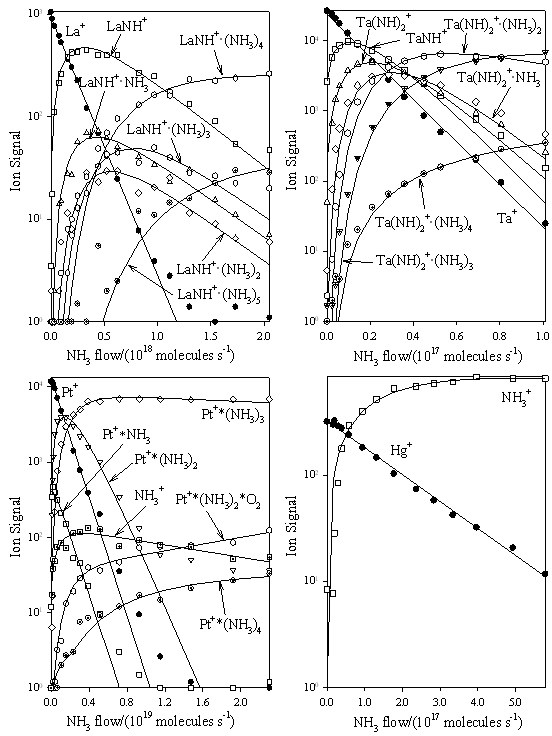
M+ + NH3 Chemistry
Periodic Table of Primary Reaction Kinetics

Figure 1. Periodic variations observed in the efficiencies, k/kc (represented as solid circles), for reactions of atomic cations with ammonia. k represents the measured reaction rate coefficient and kc is the calculated collision rate coefficient. Also indicated are the observed reaction channels and the ground-state electronic configurations of the atomic cations. The reactions of Tc+ and Po+ were not investigated.
Table 1. Rate coefficients (in units of cm3 molecule-1 s-1), reaction efficiencies (k/kc) and higher-order product ions measured for reactions of atomic ions M+ with nitrous oxide in helium at 0.35 ± 0.01 Torr and 295 ± 2 K ordered according to row in the periodic table. Products and product distributions are also included along with calculated collision rate coefficients, kc, (see text) and reaction efficiencies, k/kc.
| REACTANTS | PRODUCTS | BR | kexp | kc | kexp/kc | HIGHER ORDER PRODUCTS |
| NH3 | ||||||
| K+ | K+(NH3) | 1.00 | 1.2×10-12 | 2.3×10-9 | 5.2×10-4 | K+(NH3)2-3 |
| Ca+ | Ca+(NH3) | 1.00 | 1.2×10-12 | 2.3×10-9 | 5.2×10-4 | Ca+(NH3)2-6 |
| Sc+ | ScNH+ + H2 | 1.00 | 3.2×10-10 | 2.3×10-9 | 0.14 | SCnH+(NH3)1-5 |
| Ti+ | TiNH+ + H2 | 1.00 | 2.5×10-10 | 2.2×10-9 | 0.11 | TiNH+(NH3)1-5 |
| V+ | V+(NH3) | 1.00 | 1.1×10-11 | 2.2×10-9 | 5.0×10-3 | V+(NH3)2-5 |
| Cr+ | Cr+(NH3) | 1.00 | 5.0×10-12 | 2.2×10-9 | 2.3×10-3 | Cr+(NH3)2-3 |
| Mn+ | MnNH2+ + H | 1.00 | 2.1×10-12 | 2.2×10-9 | 9.5×10-4 | MnNH2+(NH3)1-2 |
| Fe+ | Fe+(NH3) | 1.00 | 1.7×10-11 | 2.2×10-9 | 7.7×10-3 | Fe+(NH3)2-4 |
| Co+ | Co+(NH3) | 1.00 | 2.8×10-11 | 2.2×10-9 | 1.3×10-2 | Co+(NH3)2-3, CoNH2+NH3, |
| CoNH1-2+(NH3)2 | ||||||
| Ni+ | Ni+(NH3) | 1.00 | 2.0×10-11 | 2.2×10-9 | 9.1×10-3 | Ni+(NH3)2-4, NiNH+(NH3)3 |
| Cu+ | Cu+(NH3) | 1.00 | 1.2×10-11 | 2.2×10-9 | 5.4×10-3 | Cu+(NH3)2-3 |
| Zn+ | Zn+(NH3) | 1.00 | 3.5×10-12 | 2.2×10-9 | 1.6×10-3 | Zn+(NH3)2-3 |
| Ga+ | Ga+(NH3) | 1.00 | 6.3×10-13 | 2.2×10-9 | 2.9×10-4 | Ga+(NH3)2-3 |
| Ge+ | GeNH+ + H2 | 1.00 | 5.5×10-11 | 2.2×10-9 | 2.5×10-2 | GeNH+(NH3)1-3 |
| As+ | AsNH2+ + H | 1.00 | 3.9×10-10 | 2.2×10-9 | 0.18 | AsNH2+(NH3)1-2 |
| Se+ | 1.00 | 1.1×10-11 | 2.2×10-9 | 5.0×10-3 | ||
| Rb+ | Rb+(NH3) | 1.00 | 5.5×10-13 | 2.1×10-9 | 2.6×10-4 | Rb+(NH3)2-3 |
| Sr+ | Sr+(NH3) | 1.00 | 4.9×10-13 | 2.1×10-9 | 2.3×10-4 | Sr+(NH3)2-5 |
| Y+ | YNH+ + H2 | 1.00 | 1.2×10-9 | 2.1×10-9 | 0.55 | YNH+(NH3)1-5 |
| Zr+ | ZrNH+ + H2 | 1.00 | 4.3×10-10 | 2.1×10-9 | 0.2 | ZrNH+(NH3)1-5 |
| Nb+ | NbNH+ + H2 | 1.00 | 5.6×10-10 | 2.1×10-9 | 0.27 | NbNH+(NH3)1-5 |
| Mo+ | Mo+(NH3) | 1.00 | 2.7×10-12 | 2.1×10-9 | 1.3×10-3 | Mo(NH2)0-1+(NH3)2 |
| Mo(NH2)1-2+(NH3)3 | ||||||
| Ru+ | Ru+(NH3) | 1.00 | 7.7×10-12 | 2.1×10-9 | 3.7×10-3 | Ru+(NH3)2-5 |
| Rh+ | Rh+(NH3) | 1.00 | 9.4×10-12 | 2.1×10-9 | 4.5×10-3 | Rh+(NH3)2-4, RhNH+(NH3)4 |
| Pd+ | Pd+(NH3) | 1.00 | 8.3×10-12 | 2.1×10-9 | 4.0×10-3 | Pd+(NH3)2-4 |
| Ag+ | Ag+(NH3) | 1.00 | 2.6×10-12 | 2.1×10-9 | 1.2×10-3 | Ag+(NH3)2-3 |
| Cd+ | Cd+(NH3) | 1.00 | 1.6×10-12 | 2.1×10-9 | 7.6×10-4 | Cd+(NH3)2-3 |
| In+ | In+(NH3) | 1.00 | 3.2×10-13 | 2.1×10-9 | 1.5×10-4 | In+(NH3)2-3 |
| Sn+ | Sn+(NH3) | 1.00 | 2.5×10-12 | 2.1×10-9 | 1.2×10-3 | Sn+(NH3)2-4 |
| Sb+ | Sb+(NH3) | 1.00 | 7.6×10-12 | 2.1×10-9 | 3.6×10-3 | Sb+(NH3)2-4 |
| Te+ | Te+(NH3) | 1.00 | 1.9×10-12 | 2.1×10-9 | 9.0×10-4 | Te+(NH3)2 |
| Cs+ | Cs+(NH3) | 1.00 | <10-13 | 2.0×10-9 | <5.5×10-4 | Cs+(NH3)2 |
| Ba+ | Ba+(NH3) | 1.00 | 2.0×10-13 | 2.0×10-9 | 1.0×10-4 | Ba+(NH3)2-5 |
| La+ | LaNH+ + H2 | 1.00 | 3.3×10-10 | 2.0×10-9 | 0.17 | LaNH+(NH3)1-5 |
| Hf+ | HfNH+ + H2 | 1.00 | 1.1×10-9 | 2.0×10-9 | 0.55 | HfNH+(NH3)1-5 |
| Ta+ | TaNH+ + H2 | 1.00 | 1.6×10-9 | 2.0×10-9 | 0.8 | Ta(NH)2+(NH3)0-4 |
| W+ | WNH+ + H2 | 1.00 | 9.6×10-10 | 2.0×10-9 | 0.48 | W(NH)2+(NH3)0-3 |
| Re+ | Re+(NH3) | 1.00 | 1.0×10-11 | 2.0×10-9 | 5.0×10-3 | Re+(NH3)2 |
| Os+ | OsNH+ + H2 | 1.00 | 6.5×10-10 | 2.0×10-9 | 0.33 | Os(NH)2+(NH3)0-3 |
| Ir+ | IrNH2+ + H | 1.00 | 4.1×10-10 | 2.0×10-9 | 0.21 | IrNH2+(NH3)0-4 |
| Pt+ | Pt+(NH3) | 1.00 | 5.2×10-11 | 2.0×10-9 | 2.6×10-2 | Pt+(NH3)2-4 |
| Au+ | Au+(NH3) | 1.00 | 2.5×10-11 | 2.0×10-9 | 1.3×10-2 | Au+(NH3)2-3 |
| Hg+ | NH3+ + Hg | 1.00 | 9.5×10-10 | 2.0×10-9 | 0.48 | |
| Tl+ | Tl+(NH3) | 1.00 | <10-13 | 2.0×10-9 | <5.5×10-4 | Tl+(NH3)2-3 |
| Pb+ | Pb+(NH3) | 1.00 | 5.7×10-13 | 2.0×10-9 | 2.9×10-4 | Pb+(NH3)2-3 |
| Bi+ | Bi+(NH3) | 1.00 | 7.5×10-13 | 2.0×10-9 | 3.8×10-4 | Bi+(NH3)2-3 |
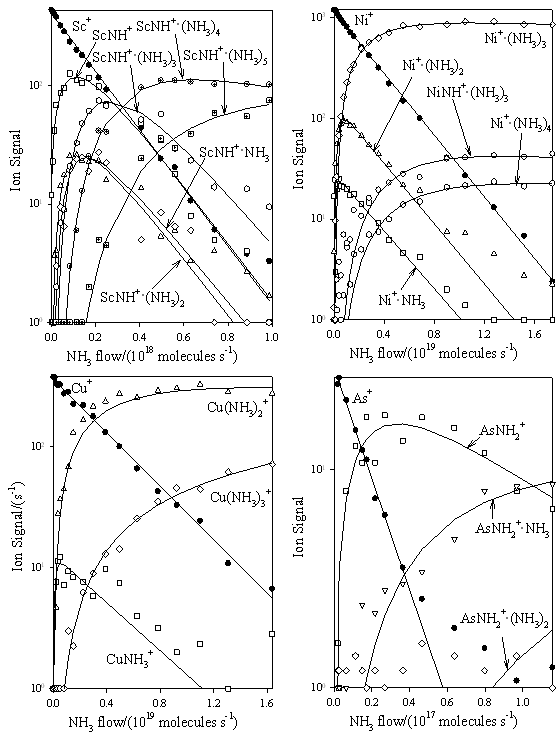
Figure 2.
Composite of ICP/SIFT results for the reactions of the first-row atomic ions Sc+, Ni+, Cu+ and As+ with NH3 in helium buffer gas at 0.35±0.01 Torr and 295±2 K.
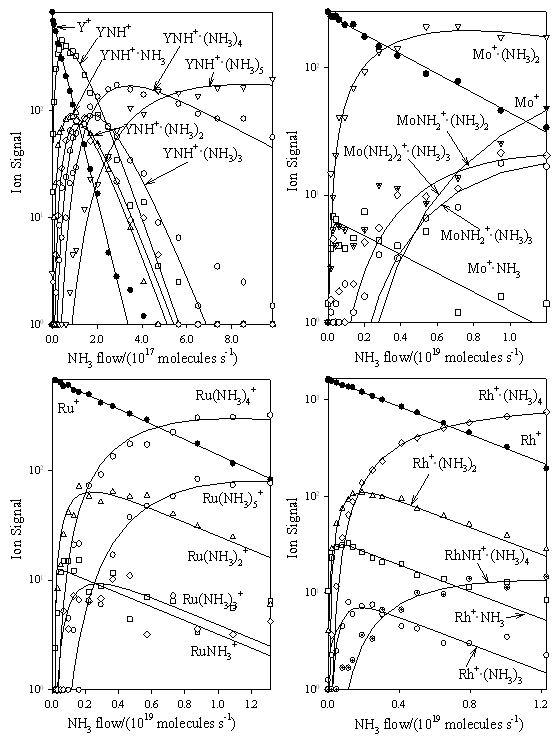
Figure 3.
Composite of ICP/SIFT results for the reactions of the second-row atomic ions Y+, Mo+, Ru+ and Rh+ with NH3 in helium buffer gas at 0.35±0.01 Torr and 295±2 K.